[Last update: November 2025]
Quick Links to Topics Covered in This Article:
INTRODUCTION • 17 Essential Elements
Using the Soil pH Chart • How a Soil Analysis Report Can Help You
How To Get Your Soil Tested
INTRODUCTION
Have you ever gone to a tree nursery and, to your glee, found what you thought to be the PERFECT tree for your home? Perhaps you saw it first in a magazine, on TV, or online, or maybe even caught it on sale at your local Walmart or Home Depot. Wherever you saw it, you impulsively purchase it for your property. In no time, you’ll have it in the ground and be taking selfies with it to brag to all your friends on social media!

After a few weeks, months, or years go by, you begin to notice it’s not looking as nice as it did when you planted it. “What happened? Why is it looking so sickly?” you wonder.
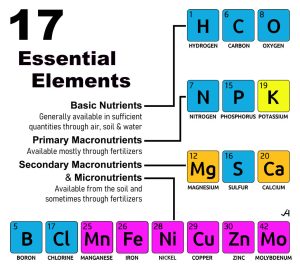
The 17 Essential Elements
Like most living organisms, a lack of any vital nutrients can have a negative health impact. Your trees are living organisms. Trees exhibit all the characteristics of life, such as growth, reproduction, responsiveness to their environment, and metabolism. They are made of cells, take in nutrients and water, and perform respiration and excretion. Therefore, KNOWING your soil can also help you to understand why existing trees may be failing in health. A soil analysis can help guide you to improve your soil management.
There are 17 essential elements for trees, based on the amounts needed, which are obtained from air, water, and soil, and are divided into macronutrients and micronutrients. Macronutrients are required in large amounts, while micronutrients, though needed in smaller quantities, are equally critical for survival and healthy growth.
- Basic Nutrients (Basic Macronutrients)
These non-mineral elements are primarily supplied by air and water, rather than the soil.- Hydrogen (H): Comprises about 6% of a tree’s dry weight. Acquired from water, it is a key component of carbohydrates and other organic compounds that are essential for growth.
- Carbon (C): Makes up about 45% of a tree’s dry weight. It is the foundation of all organic molecules and is used by trees to produce carbohydrates (a.k.a., sugars and starches) during photosynthesis.
- Oxygen (O): Also makes up about 45% of a tree’s dry weight. It is obtained from water and carbon dioxide and is vital for cellular respiration, the process that releases energy for the plant to use.
- Macronutrients
Trees require these six nutrients in relatively large amounts, which are supplied by the soil and often supplemented with fertilizers.- Nitrogen (N): Promotes strong foliage growth and green leaves because it is a key component of chlorophyll, the molecule central to photosynthesis. It is also essential for creating amino acids, nucleic acids (DNA and RNA), enzymes, and proteins. A deficiency results in yellowing (a.k.a., chlorosis) of older leaves and stunted growth.
- Phosphorus (P): Crucial for converting sunlight into usable energy, a process vital for root growth, flowering, and seed development, phosphorus also plays a central role in the transfer and storage of energy within the plant. It helps trees recover from stress and increases their overall strength.
- Potassium (K): Supports overall tree resilience by helping to regulate a tree’s water uptake and internal moisture levels, which improves its tolerance to drought, cold, and disease. It is also essential for activating the enzymes involved in photosynthesis and for strengthening cell walls.
- Magnesium (Mg): As the central atom in the chlorophyll molecule, magnesium is critical for photosynthesis. It also helps regulate the transport of phosphorus and is involved in activating enzymes for carbohydrate and protein metabolism.
- Sulfur (S): Important for synthesizing proteins, amino acids, and vitamins, sulfur is also involved in forming chlorophyll. It helps the tree resist diseases.
- Calcium (Ca): As a key component of cell walls and membranes, calcium provides the structural integrity of the plant. It promotes root and shoot growth and improves disease resistance. It is also important for regulating the transport of nutrients.
- Micronutrients
Trees need these eight soil-derived elements in very small, or trace, amounts. While needed in smaller quantities, these trace elements are critical for specific enzymatic functions. In excessive quantities, some can become toxic.- Boron (B): Important for cell wall formation, cell division, and the development of new growth, pollination, and fertilization, and also aids in the transport of sugars.
- Chlorine (Cl): Necessary for the regulation of osmotic pressure, maintaining ionic balance, vital for photosynthesis and root growth, and also helps regulate water retention.
- Manganese (Mn): This element activates enzymes involved in growth and photosynthesis, respiration, and nitrogen assimilation. It assists iron chlorophyll formation.
- Iron (Fe): Necessary for producing chlorophyll, iron functions as a catalyst in energy transfer and is a component of many enzyme systems. A deficiency causes yellowing of the younger leaves (a.k.a., iron chlorosis).
- Nickel (Ni): A component of the enzyme urease, and is necessary for nitrogen metabolism. Without it, urea can accumulate to toxic levels. Required to complete the life cycle of the tree and produce viable seeds.
- Copper (Cu): Involved in photosynthesis, respiration, and the formation of cell walls, and also helps activate enzymes and increases disease resistance.
- Zinc (Zn): Vital for enzyme function, protein synthesis, and hormone regulation, and is essential for plant growth and promoting root development, including the production of auxins.
- Molybdenum (Mo): This element is essential for nitrogen metabolism and needed for the tree to utilize nitrogen effectively, helping enzymes convert nitrates into usable proteins.
What can cause nutrient deficiencies or nutrient-deficient-like symptoms in your trees? Well… it could be any number of reasons:
-
- Poor quality tree stock
- Wrong Hardiness Zone
- Transplant shock
- Poor root establishment
- Planted too deeply
- Inadequate amounts of water
- Repetitive mechanical injuries
- Incompatible or poor soil conditions
Let’s take a few moments to consider the last item on this list: Incompatible or poor soil conditions, by examining the Soil pH Chart that follows.
One of the biggest challenges in establishing some trees in Indiana clay soils is high alkalinity and compaction. Soil pH is a measure of the acidity or alkalinity of the soil, measured on a logarithmic scale from 0 to 14. A pH of 7 is neutral; values below 7 indicate increasing acidity, and values above 7 indicate increasing alkalinity.
Soil pH is defined chemically as the negative logarithm of the hydrogen ion (H⁺) concentration in the soil solution. It is a critical indicator of soil health because it governs the availability of nutrients to plants and affects the activity of beneficial soil microorganisms. Acidic soils (typically found in high rainfall areas) have a higher concentration of hydrogen ions. Alkaline (basic) soils (common in arid regions) have a lower concentration of hydrogen ions and a higher concentration of hydroxyl ions (OH⁻).
Optimal Range: Most plants thrive in slightly acidic to neutral soils (pH 6.0 to 7.0), where nutrient availability is highest. During the planning phase of tree selection, an overlooked first step is getting a soil test. Become familiar with the following Soil pH Chart. The range from Acidic to Alkaline soils can influence the success or failure of your tree(s).
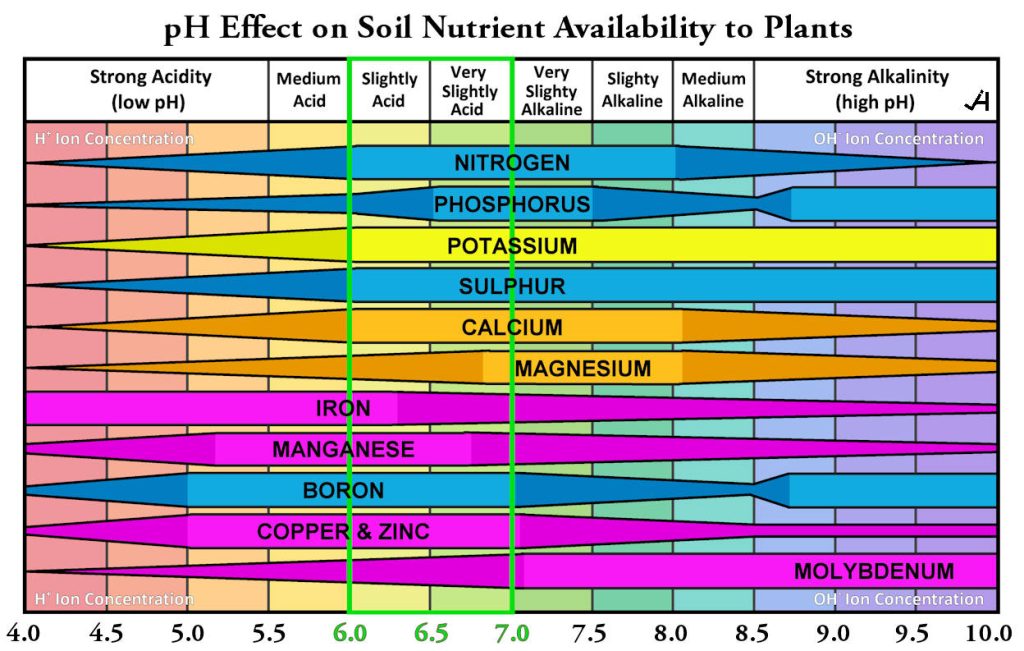
A soil pH chart is a tool that displays the soil pH scale, showing how the acidity or alkalinity of soil affects the availability of plant nutrients and the health of microorganisms.
FUN FACT: DID YOU KNOW?
When it comes to Soil pH Charts and soil guides, many focus on a scale of 4.0 to 10.0 instead of 0 to 14. Why? Because the 4.0 – 10 range encompasses virtually all naturally occurring and agriculturally relevant soil pH values. While the theoretical pH scale in water-based solutions extends from below 0 to above 14 (depending on the concentration), natural soils generally range from an extremely acidic pH of around 3.5 to a very alkaline pH of 10.0. Additionally, soils with a pH value close to 0 or 14 are exceptionally rare in nature. Such extreme conditions would require extremely strong acids or bases, which are not found in typical soil environments. Therefore, charts for gardening and agriculture aim to present the most practical information for plant growth and soil management. Since healthy plant growth is severely limited outside the 4 to 9 range due to nutrient deficiencies or toxicities (e.g., aluminum toxicity at low pH), focusing on the 0-10 or 4-10 range is sufficient for most practical purposes. NOW YOU KNOW!
This DID YOU KNOW? is based on an online article by Guodong “David” Liu and Edward Hanlon (Publication #HS1207 (March 2024), IFAS Extension)
After a laboratory analysis, a soil test report will typically show specific soil nutrient content, soluble salts, and pH level.

Collecting soil samples for lab analysis is an important step before selecting trees for planting.
NOTE: Typically, standard soil tests do not analyze for contaminants, such as bacteria, mold, fungi, herbicides, etc. Therefore, if soil contamination is a concern (say, for example, bacterial problems associated with Crown Gall disease, which affects roses, euonymus plants, willows, etc.), then more advanced analysis is needed, and that would require the help of a microbial services laboratory, which may charge a significantly higher lab processing fee.
When foliage lacks normal spring and summer coloration and displays pale or yellowish tones rather than green, it may be due to a nutrient deficiency condition known as Chlorosis.
Chlorosis is the yellowing of leaves in trees due to a lack of chlorophyll, which is caused by a deficiency in essential nutrients like iron, magnesium, or nitrogen. This impairs photosynthesis, leading to symptoms like pale green or yellowing leaves, and can progress to leaf scorching, twig dieback, and eventual tree death if untreated. The most prominent sign is leaves turning a pale green or yellow, often with darker green veins (interveinal chlorosis).
Common Nutrient Deficiencies and Contributing Factors Associated with Chlorosis:
- Iron deficiency: Typically causes interveinal chlorosis, starting on younger leaves, as the veins remain green while the tissue between them turns yellow.
- Nitrogen deficiency: Tends to cause older leaves to yellow uniformly.
- Magnesium deficiency: Often shows yellowing between the veins but may also turn the leaves bronzy-orange.
- High soil pH: Alkaline soils can make it difficult for trees to absorb iron and other nutrients, even if they are present.
- Poor soil conditions: Compacted or waterlogged soil can restrict root function and nutrient uptake.
- Root damage: Physical damage to roots from construction or disease can also cause chlorosis.
How a SOIL ANALYSIS REPORT Can Help You
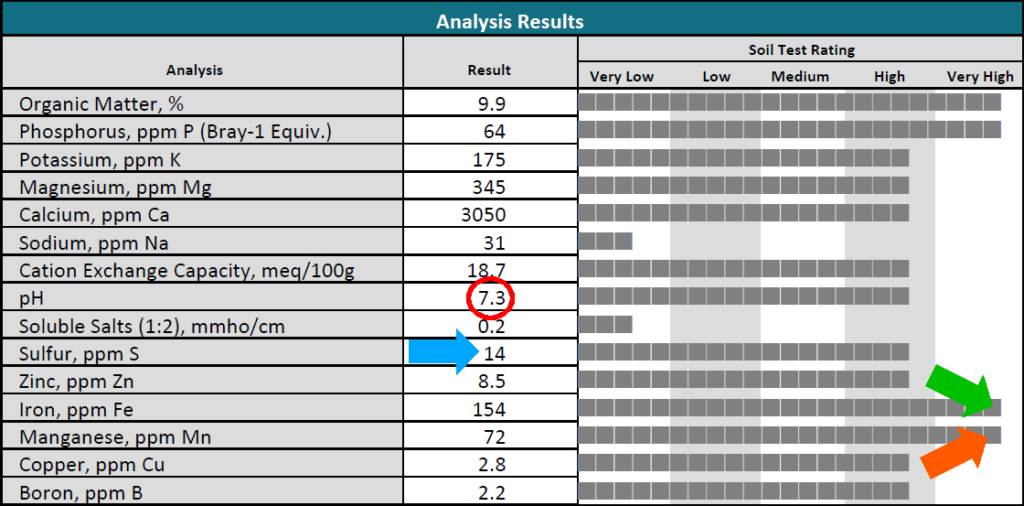
- This is where the pH rating may be an important factor to note. What is the soil pH rating? Is it too low, too high, or just about right? In reference to the aforementioned Soil pH Chart, the optimal pH range for most plants on a rating scale from 0 to 14 is between 6.0 to 7.0, with 7.0 being neutral. In the example Analysis Results report below, the pH level of the soil sample submitted is 7.3 (circled in red) and considered high.
- The soil texture (or percentage of sand, silt, or clay) determines how much Sulfur (S) content is needed to lower the pH. When soil pH is high and Sulfur is low or insufficient to reduce the alkalinity of the soil, nutrient absorption by the roots of some trees may decrease. In the soil sample below, the Sulfur reading (in parts per million (ppm); see blue arrow) is 14 ppm. This indicates that Sulfur is high, but since the pH is also high. This could indicate a problem related to soil texture.
- Should you add more Iron (Fe) into the soil for your yellowing Pin Oak (or River Birch or White Pine, etc.) to help green up the foliage? Well, if the soil in this sample report above came from the property with those trees, we can see that the soil already has a very high concentration of Iron (see green arrow) at 154 ppm. Therefore, additional Iron is likely unnecessary.
- The same may apply if we were trying to resolve a Manganese (Mn) deficiency in a yellowing maple tree to help restore summer season color to the foliage. According to the Analysis Results example above, Manganese is 72 ppm is considered very high. (See orange arrow)
HOW TO GET YOUR SOIL TESTED
We highly recommend c
The laboratory we use, for example, A&L Great Lakes Laboratories, is located in Fort Wayne, IN. We rely on them to deliver comprehensive soil analysis for client trees that suggest soil-related symptoms or when they desire to plant new trees.
Getting your soil tested is something you can do yourself:
- Use the “you can do yourself” link above and print and fill out the soil submittal form.
- Choose the “Soil – Home & Garden” form on their webpage. (The actual soil sample submittal form contains instructions at the bottom of the form on how to collect & submit soil samples.)
- On the form, be sure to select to have a COMPLETE test, which costs $30.00. (See sample submittal form.)
- Choose 3 fertilizer recommendations. Since trees are always an important factor, ensure “118-Shade Trees” is circled and then choose two other categories, such as “110-Fruit Trees” and “115-Evergreen Shrubs”. (The latter two will cover you in case you plan to plant flowering crabapple trees or evergreen trees/shrubs in the future, but select whatever applies. Your soil will not change that much over the next several years, unless you have significant soil changes performed, as mentioned earlier.)
- After the lab testing is completed, they will email you a copy of their analysis report. If you retain an arborist’s PHC services, then you should make a copy of this report available for their records as well. The more information you provide, the better an arborist can help diagnose problems in your landscape!
Note: If you need soil sample collection supplies like a submittal bag, shipping box, etc., they sell these supplies on their site as well. We use USPS to ship our samples to the lab, but you can use whatever delivery service is appropriate.
With soil test results in hand… ARE YOU READY TO PLANT A TREE?
Follow this link ![]() HOW TO PLANT A TREE!
HOW TO PLANT A TREE!
Related articles:
- Soil and Water pH – Part 1: What is pH? https://ucnfa.ucdavis.edu/news/soil-and-water-ph
- Soil and Water pH – Part 2: How is nutrient availability affected by pH? https://ucnfa.ucdavis.edu/news/get-cultured-part-2-soil-and-water-ph
- Soil and Water pH – Part 3: How do fertilizers affect pH? https://ucnfa.ucdavis.edu/news/soil-and-water-ph-part-3
- Understanding and Applying Chelated Fertilizers Effectively Based on Soil pH http://edis.ifas.ufl.edu/hs1208
- Chelates – What are they and What do they do? (PDF) https://www.gchydro.com/pdf/GreenCoast%20Hydroponics-IS-NUT-0413-The%20Science%20Behind%20Chelates.pdf
- Predicting Soluble Nickel in Soils Using Soil Properties and Total Nickel https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0133920
FUN FACT: DID YOU KNOW?
Chlorine and Nickel are often not explicitly represented on typical, simplified soil pH charts because their availability is predictable from the behavior of other nutrients already on the chart, or their concentrations are usually sufficient across most pH ranges. Unlike many cations whose availability changes drastically with pH due to precipitation at higher pH levels, chloride is highly mobile in the soil solution, and its availability is generally not a limiting factor or strongly tied to pH fluctuations in the same way as other nutrients are. Its behavior is similar to other anions like nitrate (NO₃⁻), which also do not typically appear on these charts for the same reason. Nickel availability in the soil is similar to other divalent cations like copper (Cu), zinc (Zn), iron (Fe), and manganese (Mn). These metals become less available at high pH and more available at low pH, to the point of potential toxicity. Since these other metals are already on the chart to illustrate this general trend for micronutrient metal cations, a separate line for nickel is often considered redundant in a simplified visual representation. NOW YOU KNOW!
This DID YOU KNOW? is based on an online article by Guodong “David” Liu and Edward Hanlon (Publication #HS1207 (March 2024), IFAS Extension)